Resolves diagnostic uncertainty of indeterminate thyroid nodules
Molecular tests for indeterminate thyroid nodules are the most advanced and accurate diagnostic tool to avoid unnecessary surgeries.
The molecular test that offers the highest diagnostic accuracy.
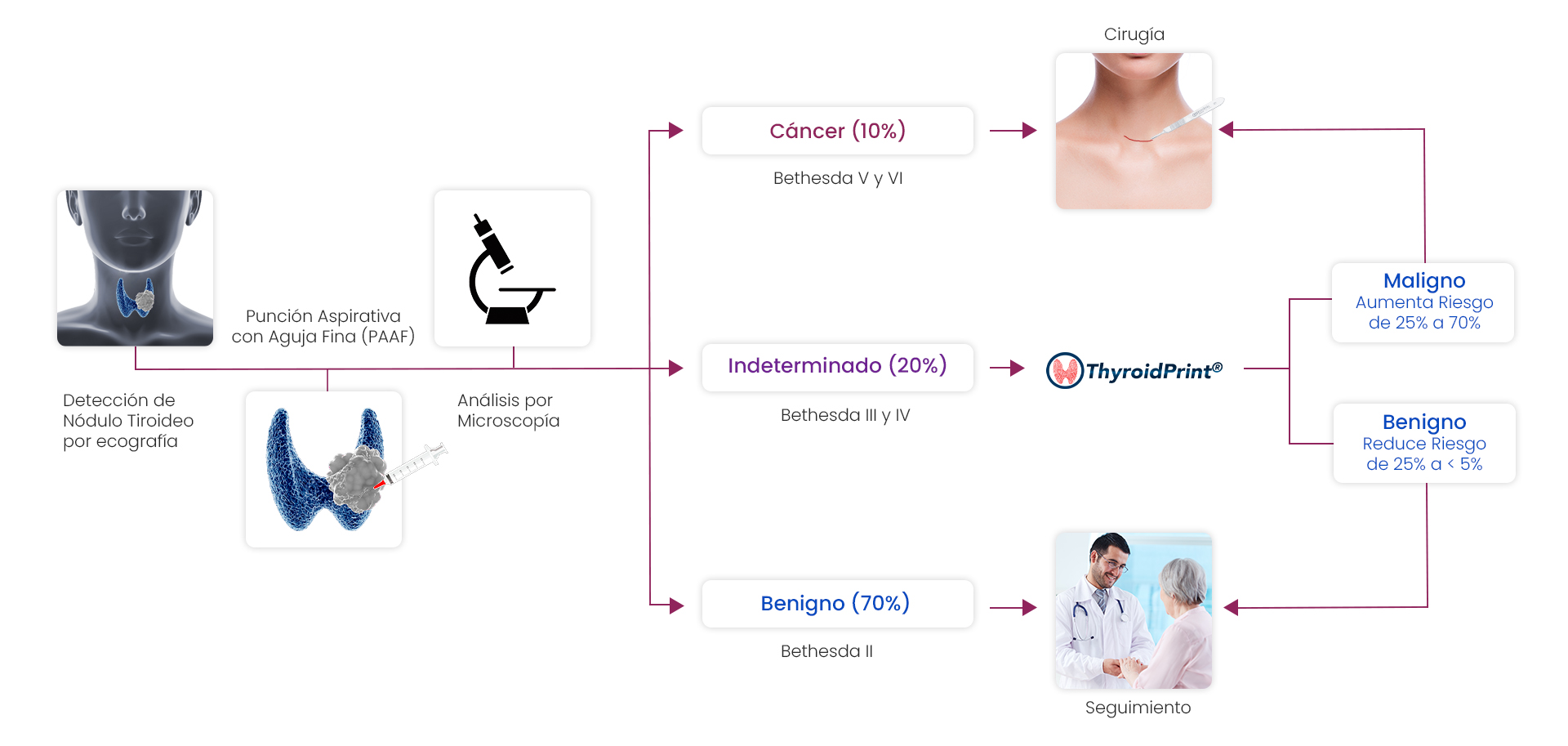
Patients who have undergone a thyroid FNA biopsy which has been reported as indeterminate (Bethesda III or IV) have a risk of malignancy ranging from 15% to 35%. ThyroidPrint is a test allows resolving this diagnostic uncertainty by predicting with 95% negative predictive value whether the indeterminate nodule is benign or not.
High diagnostic accuracy
Value
Indicates that the probability of the nodule being malignant decreases from 25% to less than 5%, allowing follow-up to be recommended as an alternative to surgery.
Value
Indicates that the probability of the nodule being malignant increases from 25% to 78%. In these cases, surgery is recommended.
Indicates the percentage of benign cases that would avoid unnecessary surgery.
GENOMIC DIAGNOSIS BY THYROIDPRINT
The largest multicenter study conducted by a molecular thyroid test.
ThyroidPrint has been clinically validated in the United States and Chile. This ethnically diverse, multicenter (15 centers), prospective, double-blind stud recruited 4,100 patients, showing equivalent clinical performace regardless of patient ethnic diversity.
Clinical Validation

4,100 Patients










900 Patients







Scientific Evidence
Check out the scientific evidence supporting the high performance of ThyroidPrint here.
SUBSCRIBE
Multicenter Validation
Leave us your details and we will send you the papers, latest news, and publications about the ThyroidPrint test.